Other people have described the health effects, so I'll describe the chemistry. Fats are made of long chains of carbon atoms surrounded by hydrogen atoms attached to a "head", which is made of other elements or structures. Carbon atoms normally can make a total of 4 bonds. Hydrogen atoms can make 1 bond.
Carbon being able to make 4 bonds means that in the chain of carbon and hydrogen atoms in fat molecules, each carbon atom makes a bond with the carbon atom before it in the chain, a bond with the carbon atom after it in the chain, and then bonds with two hydrogen atoms separately off to the side. This makes a total of 4 bonds. If all of the carbon atoms in the chain are like this, that's "saturated fat", because the chain of carbon is completely "saturated" with hydrogen atoms.
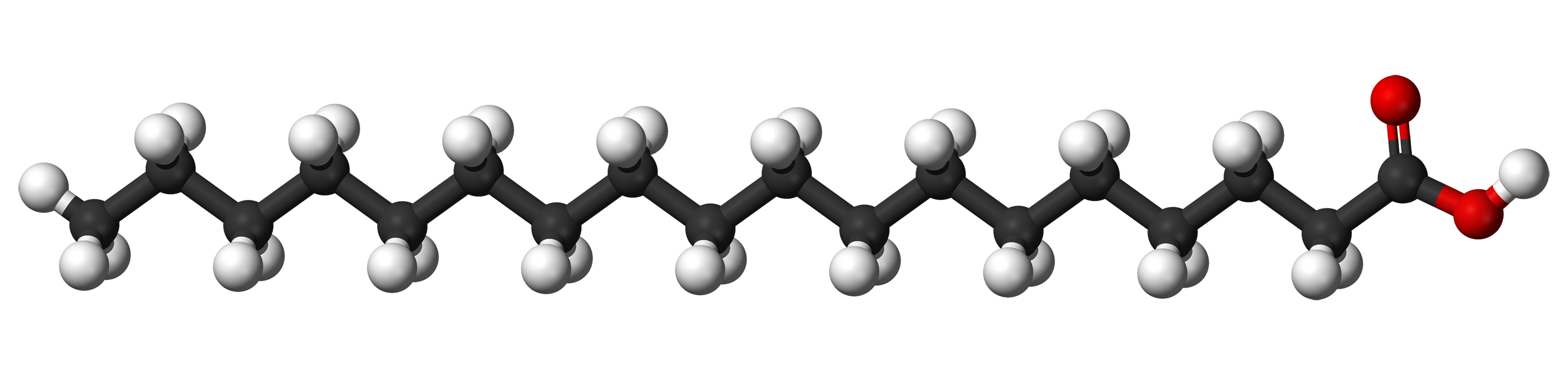
(Hydrogen atoms are white, carbon atoms are black, oxygen atoms are red)
Saturated fats have the often desirable property of being able to be tightly packed together, and thus are typically solid at room temperature. Butterfat is mostly saturated fat.
However, carbon atoms can also make a double bond with other carbon atoms. If a particular carbon atom in the chain makes a double bond with the carbon atom before it, it could cause a bend in the chain of carbon atoms. In that case, it also means that those particular carbon atoms in the chain that have formed a double bond with each other only have 1 available bond left (after also forming a separate single bond with the carbon atom before or after it), so it can only bond with one hydrogen atom. These are, therefore, called "unsaturated fats", and because they don't pack together easily, they are typically liquid at room temperature.
If there is a single double bond in the chain, it's a monounsaturated fat.
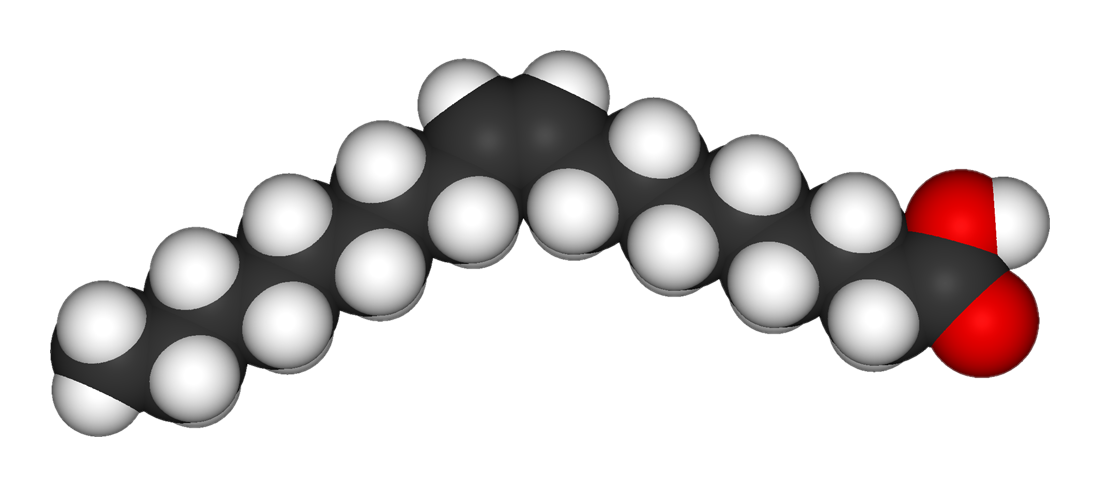
If there are two or more double bonds, it's a polyunsaturated fat.
Notice how the hydrogen atoms connected to the double-bonded carbon atoms in unsaturated fats can be connected to either the same side or the opposite sides of the two hydrogen atoms. If they're on the same side, they are called cis-unsaturated fats. If they're on opposite sides, they are trans-unsaturated fats, or trans fats in short.
This is oleic acid, a cis monounsaturated fat commonly found in many vegetable oils:

While this is vaccenic acid, a trans-monounsaturated fat. It is found naturally in butter and human milk and is not particularly bad for you:
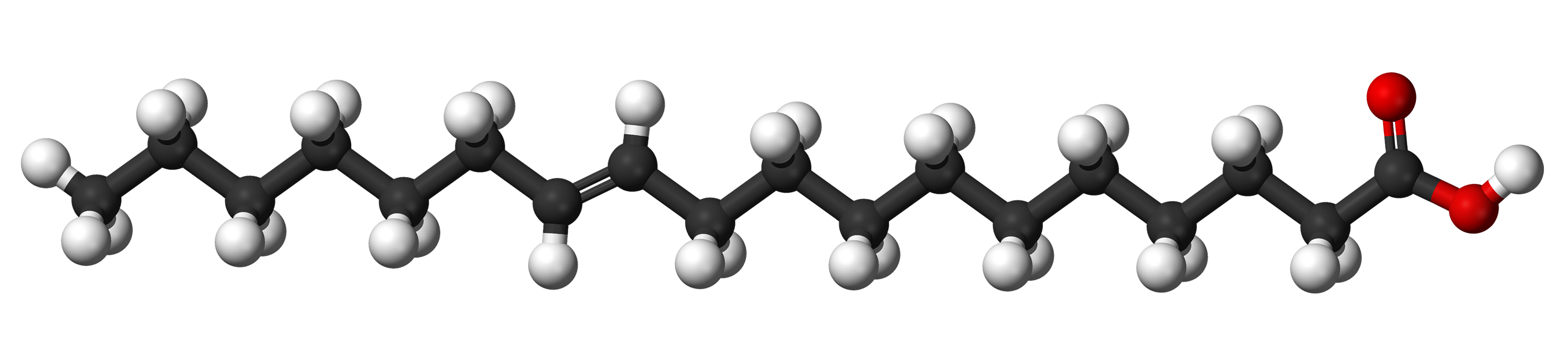
Note that this is NOT the same picture as the one I showed for saturated fat. The 7th and 8th carbon atoms from the left are double-bonded and, therefore, are each missing a hydrogen atom. The one remaining hydrogen atom on each is bonded on opposite sides.
Note that trans-unsaturated fats are also pretty straight. This means that they can also pack together with saturated fats to make a solid product at room temperature.
"Hydrogenation" is the process of adding hydrogen to unsaturated fats to saturate them. This means that liquid oil can be processed into a solid product. That's how margarine and shortening are made. In previous years, partially hydrogenated oils that weren't fully hydrogenated could leave substantial quantities of trans-unsaturated fats left in the product, but after health concerns, many countries' food safety authorities banned these artificial trans fats. Fully hydrogenated fats consist of only saturated fats since they have been "fully" hydrogenated, and that is what food manufacturers have been doing instead.