Not sure how I managed to never hit this species with UV. I would describe the colour as a bright, hot, lipstick pink. I am unsure if this lichen is actually fluorescing or if something else to do with how the pigments show up under UV light - maybe @[email protected] would know. Picture doesn’t quite do it justice.
You are pointing a UV lamp at it which probably sends out 365 nm or 395 nm photons. The lichen is shooting back photons with a broad range of wavelengths, and a lot of ~600 - 750 nm ones (red). So, the UV photons had to be "captured" by some molecular system, the system dissipated some energy, and then re-radiated some of these longer-wavelength photons.
The general term that covers the many different possibilities is "photoluminescence". In this case we can say for sure that the lichen exhibits "UV-induced photoluminescence", because it is re-emitting lower energy (longer-wavelength) photons. It is common to make the connection "photoluminescence" = "fluorescence", but technically fluorescence makes specific claims about how the light is re-emitted (singlet -> singlet emission), and it is not the only luminescence process. Other examples of luminescence are phosphorescence from a triplet state and luminescence via charge-recombination. So, to call it "fluorescence" in the strict sense we need know what the exact pathway is.
That said, when it comes to biological pigments fluorescence is generally the most common pathway. Triplets that live long enough to produce light are generally undesirable as they can react indiscriminately with molecules inside of the cell as well as produce reactive oxygen species, and good phosphorescent materials often combine metals and heavy atoms that are not as abundant in living tissue.
So, knowing nothing else, and seeing that red light comes out when you shine UV/blue light on a lichen, it is generally fair to call it "fluorescence".
Now, if we discuss this specific lichen... I have looked it up and it does get interesting! Do you have it with you? I suspect that its fluorescence might be different during the day than during the night.
I can find online two significant fluorescent components: parietin, which produces the fluorescent yellow pigment, and Chlorophyll a/b, which produces red fluorescence. There is an interesting paper exploring the idea that one functional purpose of parietin's fluorescence is that it can transfer energy to the algae to boost their photosynthesis. Their conclusions in the paper is that the idea is not supported by the evidence, so, a "negative result". It is a fun example of the type of research that is performed in photobiology and also an example to show that even negative results can be interesting enough to be published!
As for the difference between day and night - if what you see is a combination of the fluorescence of parietin and chlorophyll, then the color might change with the day/night cycle. Photosynthetic organisms regulate the flow of excess photon energy towards a safe non-radiative dissipation pathway in response to light. This is called the non-photochemical quenching pathway, and during the day this pathway tends to be active. During the night there is little light, and so this protective pathway shuts-off. This allows more of the absorbed photon energy to flow into the radiative fluorescence pathway, increasing the red fluorescence. You can actually see this easily with plants - you can dark adapt a leaf and then compare its fluorescence with that of a leaf that is being exposed to a bright light. The dark-adapted one will usually show significantly more red fluorescence.
This time you did ask, so I won't apologize for my essay 😆 But I am a bit sorry I didn't have the time to make it shorter.
No. I think they both lose more than they gain here. It doesn't make sense as a strategy. Ego clash is a simple explanation.
Too bad you don't get to bring your equipment, but at least you will get to see them :D Good luck finding some wild ancestors!
Great find, congratulations!!
Enjoy your holidays!! 🕊️
Hi! I’ve looked through /r/shittyaskscience and I think it leans too far into jokes with very little actual science content. The idea behind mander is to support specific, niche science-related communities, so a general joke-focused community doesn't really fit.
For 'science_memes', the mod is a very capable superstar and I agree with their vision of memes as a laid-back way to connect people to science. It’s plausible that a community like 'shittyaskscience' could achieve something similar, but honestly I think science_memes already covers that space well.
As for !askscience - it simply hasn’t been created yet. It would be more fitting than 'shittyaskscience', but I still prefer encouraging people to ask lichen questions in the lichen community, mushroom questions in the mushroom community, chemistry questions in the chemistry community, and so on. I support content flowing toward niche communities rather than having a centralized place for general questions. A general community would be more popular, but popularity isn’t a goal, and it works against the underlying philosophy. Niche spaces may be smaller, but they offer much better signal-to-noise for building meaningful connections.
Unfortunately the article is behind a paywall... But I am curious, does this mean there won't be any new Cortex-Mxs microprocessors?
The summary says that Arm wants to "enter the chip design space". They weren't doing this already?
And, is the prospect of a proprietary chip an exciting tease when pitted against an open standard one? I am excited about RISC-V microprocessors precisely because they rely on an open standard, so I am curious to see what their angle here is. I tried to find a non-paywalled source but I couldn't find one.
Solar Maximum happens every 11 years or so, right? I will keep paying attention!
Wow, that is spectacular!
I bought a National Instrument's data acquisition card (PCIe-6535B) not knowing that National Instruments is not very Linux-friendly and I was not able to get it working. At least it was a used card so I did not pay to much for it, but I learned my lesson not to assume compatibility.
Once I also used 'rm -rvf *' from my home directory while SSH'd into a supercomputer (I made a syntax error when trying to cd into the folder that I actually wanted to delete). I was able to get my data restored from a backup, but sending that e-mail was a bit embarrassing 😆
Sal
0 post score0 comment score






















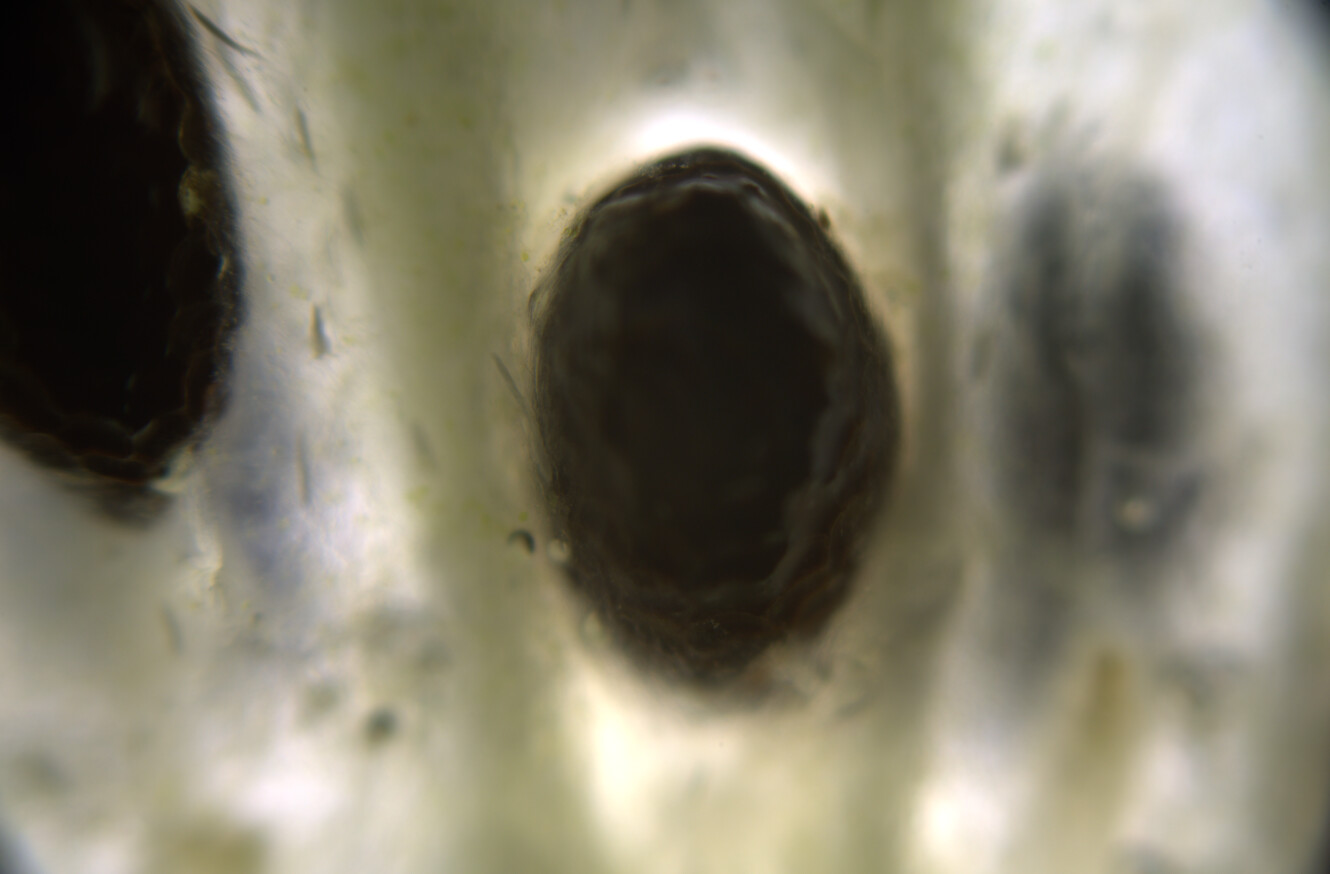

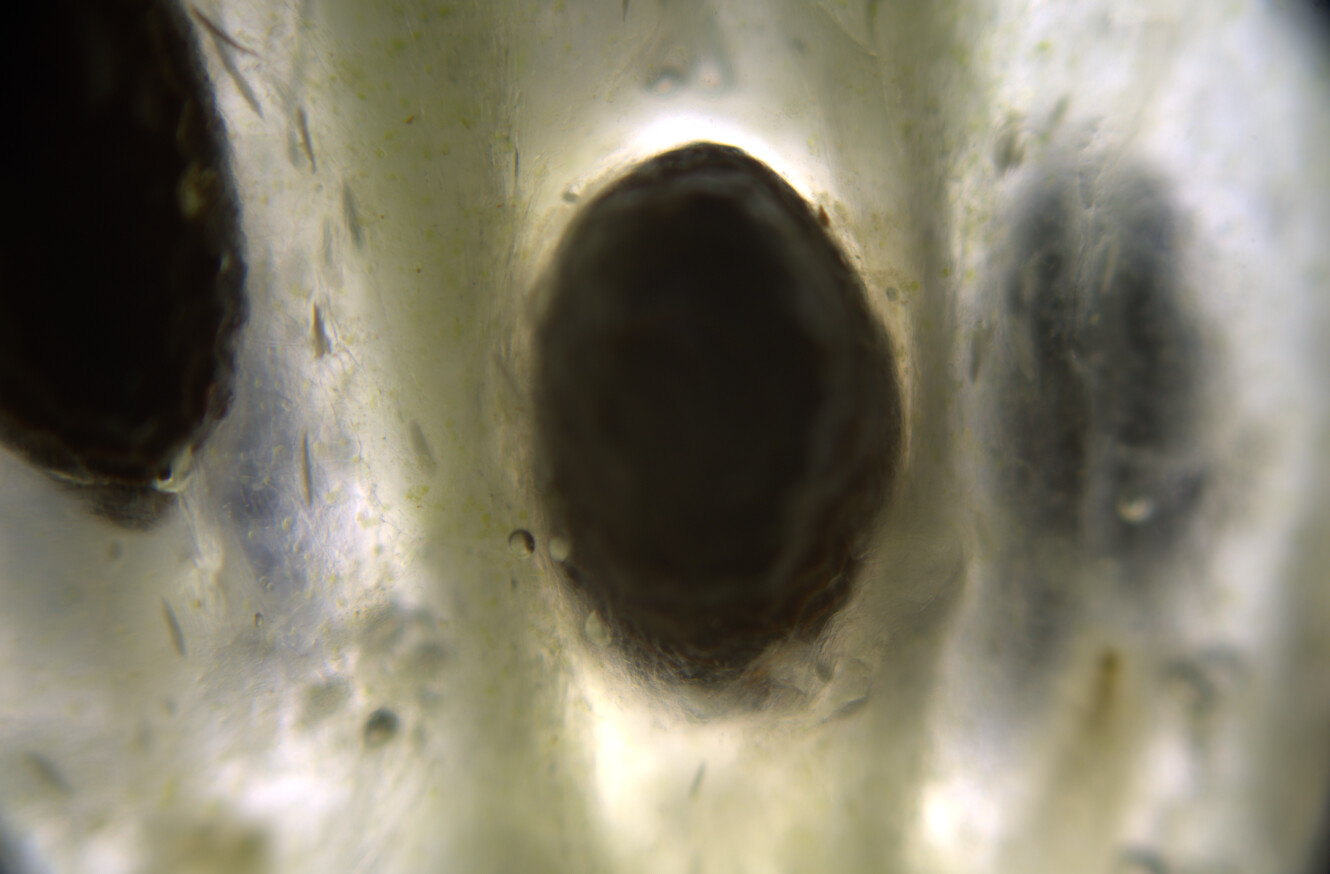
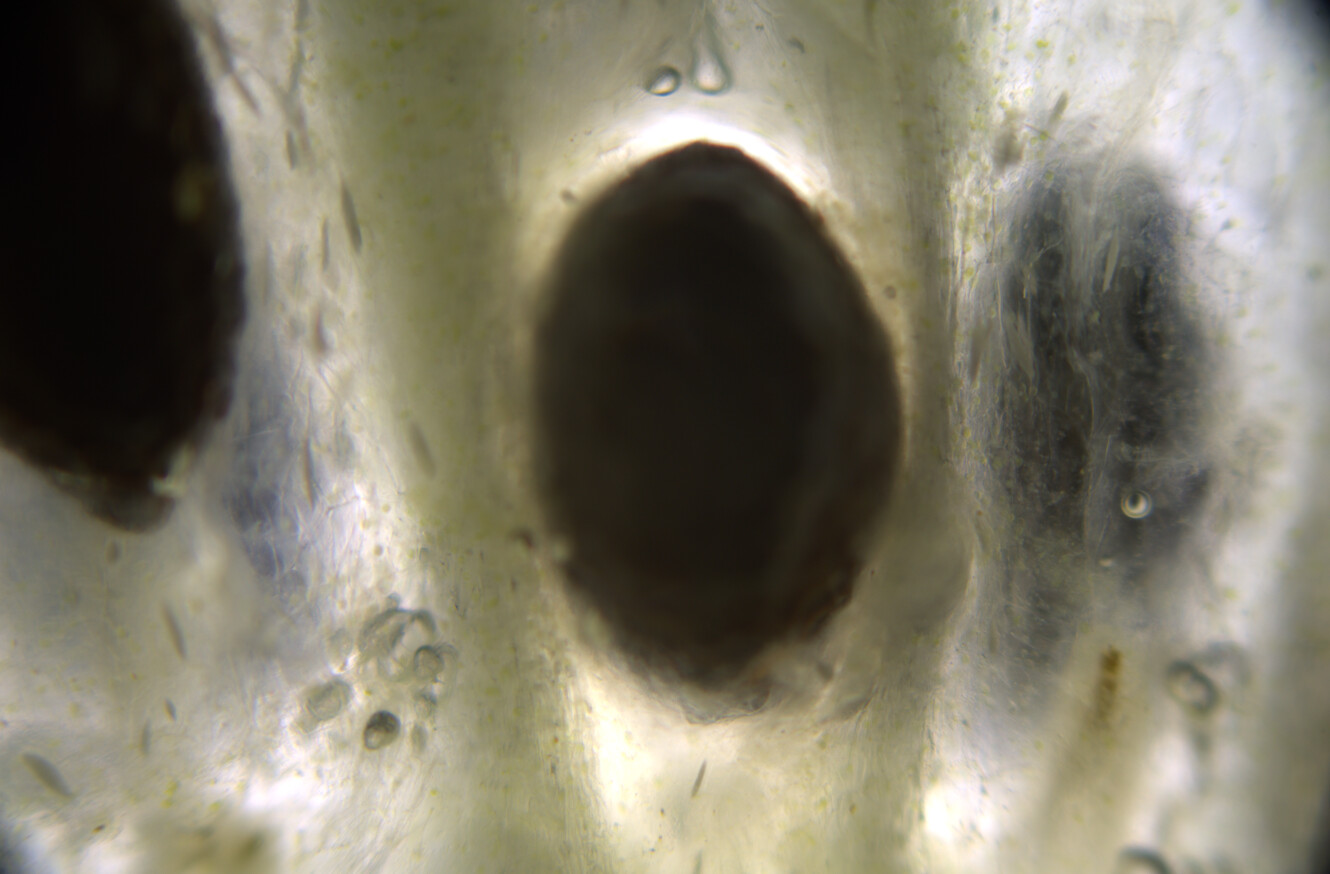











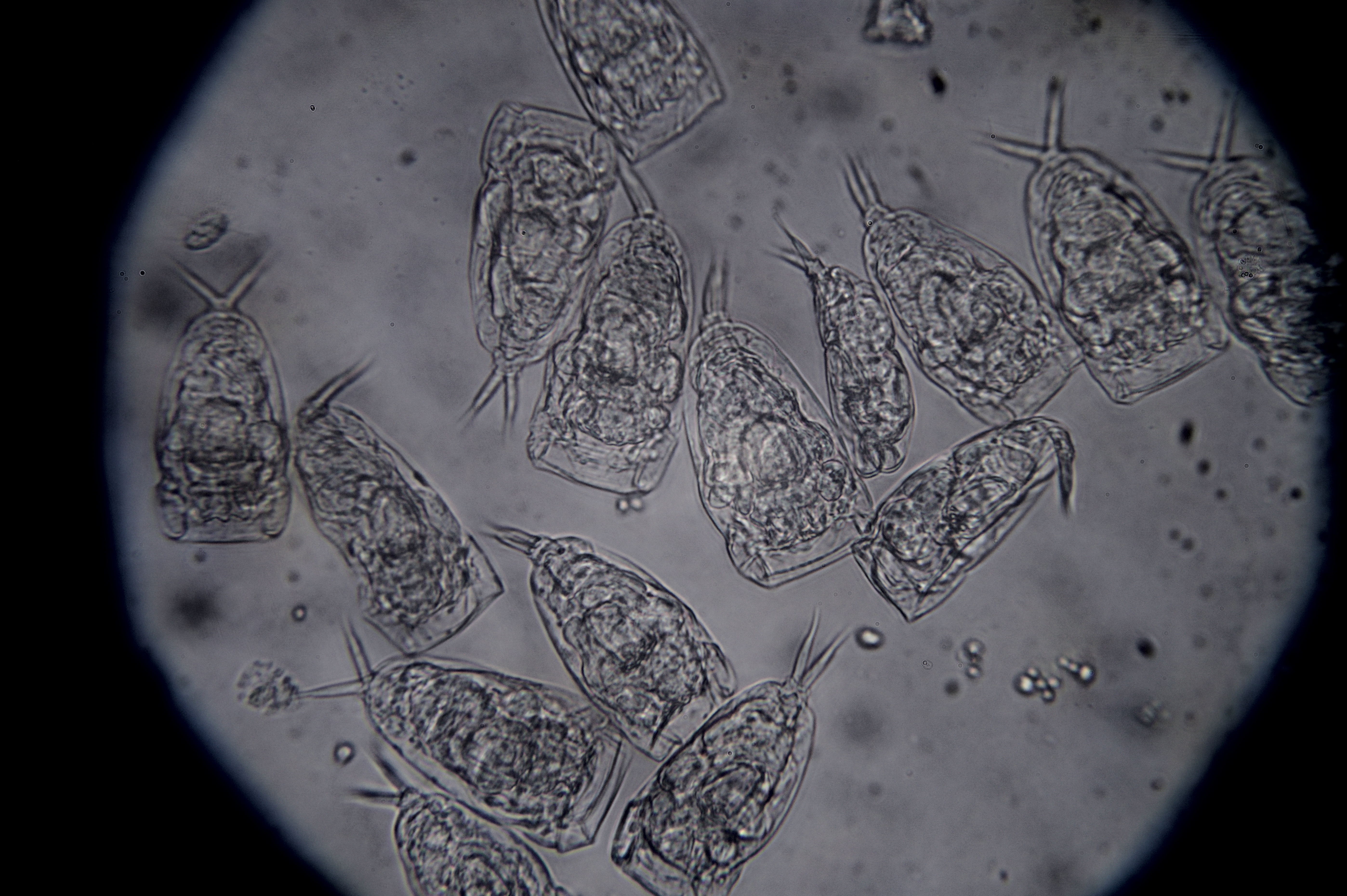
Hello ✌️ 😄