this post was submitted on 22 Sep 2024
65 points (100.0% liked)
NASA
986 readers
19 users here now
Anything related to the NASA (National Aeronautics and Space Administration); the latest news, events, current and future missions, and more.
Note: This community is an unofficial forum and is unaffiliated with NASA or the U.S. government.
Rules
- Be respectful and inclusive.
- No harassment, hate speech, or trolling.
- Engage in constructive discussions.
- Share relevant content.
- Follow guidelines and moderators' instructions.
- Use appropriate language and tone.
- Report violations.
- Foster a continuous learning environment.
founded 1 year ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
That’s not how what works? Lumps of rock of varied composition burning up aren’t going to produce the same effects, gram for gram, as lumps of a majority of high-purity aluminium. There is little evidence that meteorites produce specifically high levels of aluminium oxide particles when entering the atmosphere (at least that I can find, I’m not dogmatic about this, I just can’t find any actual evidence that disproves this idea. I wouldn’t mind being wrong!) Even the base estimates seem to suggest that the AlO particles will remain in the upper atmosphere for decades. AlO is proven to be a catalyst for ozone depletion, there’s quite a lot of research about that in relation to rocket exhaust gas.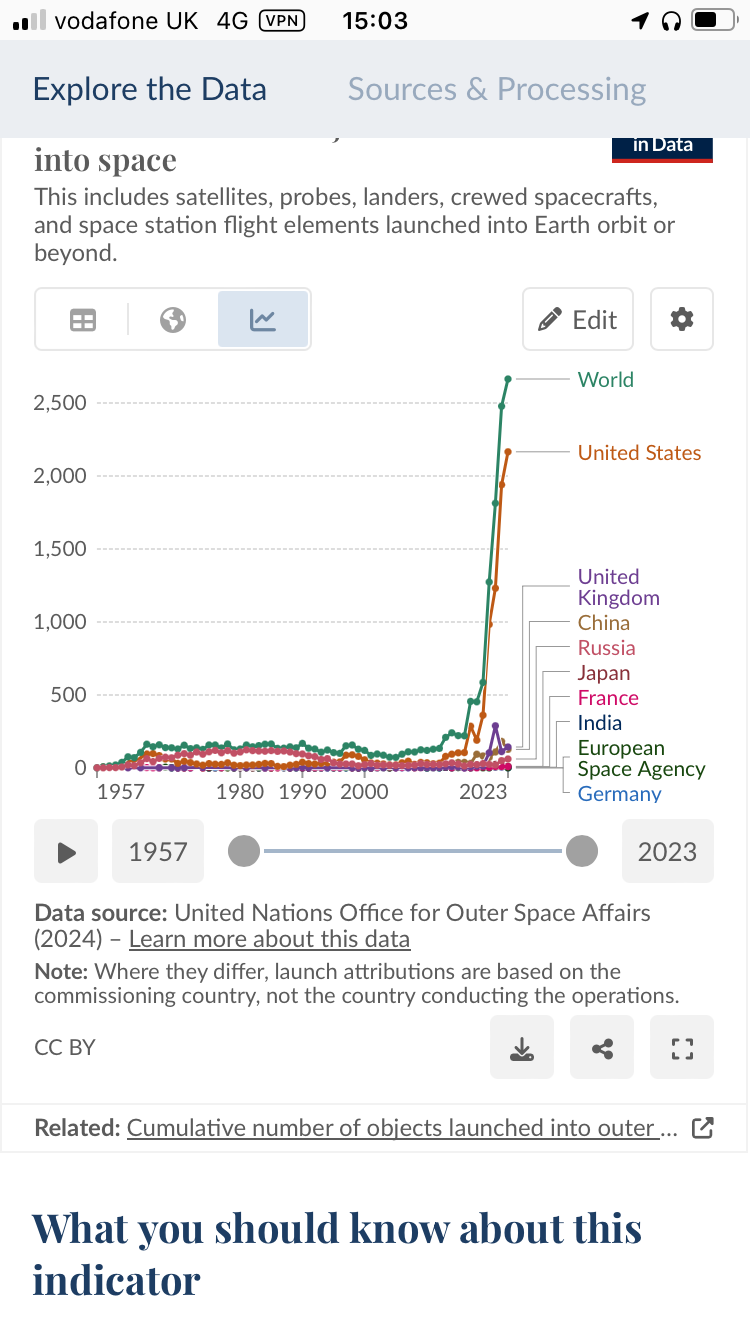
The numbers of launches, satellites, and deorbits are rising pretty rapidly, so “launching shit for decades” doesn’t really mean as much as you think -
I was pulling the number from your own paper you presented...so is your paper wrong then?