222
Boeing's Starliner successfully docks with space station after challenging rendezvous
(www.cbsnews.com)
Share & discuss informative content on: Astrophysics, Cosmology, Space Exploration, Planetary Science and Astrobiology.
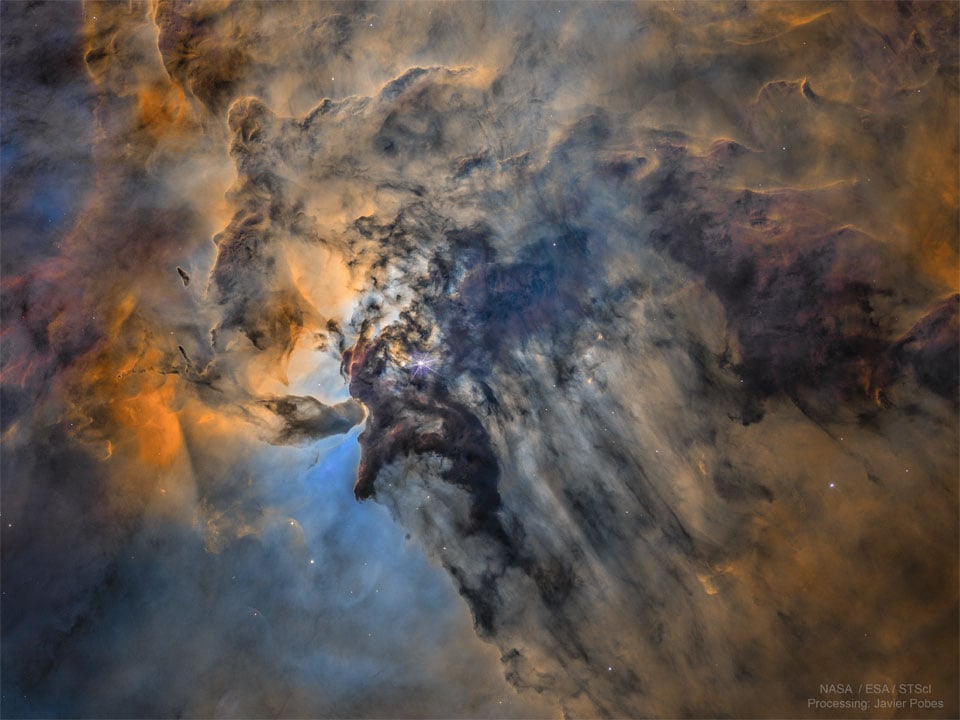 The Busy Center of the Lagoon Nebula
The Busy Center of the Lagoon Nebula
🔭 Science
🚀 Engineering
🌌 Art and Photography
Other Cool Links
I think helium is harder to contain. Although hydrogen is lighter atom, it is also a larger atom due to lower nuclear charge (Z effective). Hydrogen is also a diatomic molecule, whereas helium is a single atom. I think the only thing helium has going for it is that it doesn't easily dissolve in and embrittle metals. But helium can fit through any gap hydrogen can.